GIVE A NEW HOPE
KINETO Lab’s expert team manages the whole process of drug development from target identification and validation through high throughput in vitro screenings to in vivo animal models studies
IN VITRO – IN VIVO – EX VIVO
Our mission is to enhance the quality of drug discovery and developmental concept of our partners by providing high quality services on various tumor models which allow you to design the most suitable study to prove a concept of your research, in order to reach the clinical stage faster
Patient-derived tumor xenografts (PDTX) as clinically relevant models reflect tumor microenvironment of the patients, improving the reliability of translational research and predictability of clinical therapeutic response
15 years experienced cancer research biotech
We are a biotechnological research and CRO company which aims to use scientifically founded strategies to identify and validate new drug targets and use preclinical models for testing and developing mainly new anticancer and other anti-disease technologies and treatments.
Our company can fast, cost-effective and accurate prove a developmental concept of your research.



about us





research& development
DRUG DEVELOPMENT
Given the still dismal overall prognosis for cancer patients, the need is evident to discover and develop novel targeted and personalized anticancer therapies, where we are contributing in numerous self- and grant- funded projects
PDTX MODELS
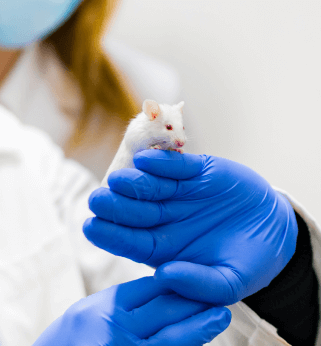
We successfully developed our system to establish, maintain and archive PDTX models in order to provide numerous options to our partners to use the most reliable preclinical models for in vivo drug testing
TARGET IDENTIFICATION

KINETO Lab is discovering novel molecular targets of tumor cells, leading to effective therapeutics development. New homing domains can selectively deliver the drugsh to its site of action
ANTIBODY DEVELOPMENT

KINETO Lab intends to expand its existing tissue culture and animal- based infrastructure on the way that it can continually be able to isolate larger amount of antibodies
DIAGNOSTICS

We are developing detection methods and devices by which it is possible to detect and capture a specific cell type using combined markers in order to improve cancer diagnosis and prognosis
PARTNERSHIP
ARE YOU WILLING TO HAVE A TRUSTFUL PARTNER AND MAKE SUCCESSFUL COLLABORATION?
If you would like to become our partner, please contact us.
SERVICES
KINETO Lab is an acknowledged expert providing tailored and unique methods to identify and validate new drug targets and to evaluate in vitro and in vivo activity of anticancer drugs in the preclinical drug discovery.
IN VITRO
KINETO Lab offers and provides wide range of in vitro preclinical services on various tumor models which allow you to determine the activity of your compound, select the best compound and develop your therapeutics
EX VIVO
We provide tumor and organ samples, which can be routinely processed, characterized and evaluated with validated pathological, biochemical and histological analysis methods supporting your research
IN VIVO
Demonstrating the antitumor activity of a novel agent in animal models is important step in preclinical drug development, therefore we propose in vivo preclinical models which can be used per se or associated with imaging modalities to permit the evaluation of the efficacy of newly developed therapeutics
immuno-oncology
In order to study immunology aspects of cancer KINETO Lab provides in vitro and in vivo models suitable to prove your developmental concept
CONSULTANCY


Nowadays, polyhistorians in the classical sense no longer work effectively. Good quality scientific work requires cooperation between representatives of different disciplines.
We can provide you with assessment,
advice and ideas on biological issues
If you are a chemistry-related research laboratory involved in drug development, and if you are planning a new project, or already have ongoing projects where the biological aspects of the topic and feasibility and rationality arise, we can provide you with assessment, advice and ideas on biological issues to put your research on the scientific or business map.

CONSULTANCY

We can provide you with assessment,
advice and ideas on biological issues
VETERINARY SERVICES
PERSONALIZED ANTI-CANCER VACCINE FOR VETERINARY USE
A fully personalized therapeutic vaccine made from the animal’s own tumor tissue, designed to activate a targeted immune response using safe, evidence-based, veterinarian-focused biotechnology
DRUG-EFFICACY TESTING FOR BETTER THERAPEUTIC DECISIONS
Real-time drug-efficacy testing on the patient’s own tumor cells, empowering veterinarians to choose the most effective, evidence-based treatment for each animal
PRODUCTS
Additionally, we offer different samples from in vitro and in vivo experiments, for your further analyses.
We can prepare and ship different samples from in vitro and in vivo experiments, for your further analyses.
Additionally, we can perform the molecular biology analysis service in our lab, providing you final results.
NEWS
04/11/2025
Published a study where Safe Delivery of a Highly Toxic Anthracycline Derivative through Liposomal Nanoformulation (LiPyDau) achieves Complete Cancer Regression, in Molecular Cancer journal
KINETO Lab announcing that research about how safe delivery of a highly toxic anthracycline derivative through liposomal nanoformulation achieves complete cancer regression, was published in Molecular Cancer journal. In this study, where was used our patient-derived tumor xenograft (PDTX) model of invasive lung adenocarcinoma, it was developed LiPyDau therapeutic with promising and highly effective therapeutic approach for combating drug resistant cancer and potential use in the clinic.
09/07/2025
KINETO Lab’s PDTX Model of BRAF V600E-mutant Melanoma was used in the study on how Realigned Transsulfuration Drives BRAF-V600E-Targeted Therapy Resistance in Melanoma, published in Cell Metabolism journal
06/03/2025
The study about targeting tumor hypoxia and immunoblockade to improve breast cancer therapy at EACR 2025 Congress, June 16th – 19th, 2025, Lisbon, Portugal
18/02/2025
Published a study about WISIT Vaccines Based on IL-31-derived Peptides as a Novel Therapeutic Approach for Chronic Pruritic Dermatoses, in PLoS One journal
CONTACTS
If you have any question regarding our research, services or PDTX models, and if you want to get in touch with us, fill the form or send us an e-mail on in**@*******ab.hu.
We are always open for your CV and application in case you would like to work in an innovative, dynamically growing cancer research-related biotech company in the future. If you think you could fit in KINETO Lab’s team, please contact us.






